Three cough syrups recalled, ordered to stop production: Drug regulator CDSCO informs WHO
Fri 10 Oct 2025, 01:12:18
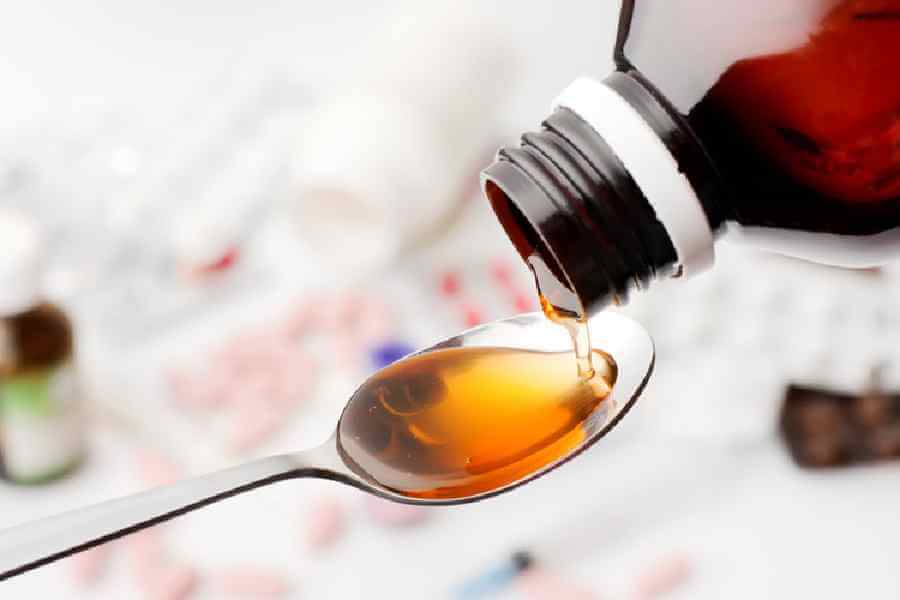
India’s drug regulator CDSCO on Thursday reposnded to the World Health Organisation (WHO) that three cough syrups -- Coldrif, RespifreshTR and ReLife -- have been recalled and manufacturers have been ordered to stop their production. The CDSCO has also informed the global health agency that none of the products were exported from India.
Earlier, the WHO had sought to know from Indian authorities whether the cough syrup linked to children deaths in the country was exported to other countries.
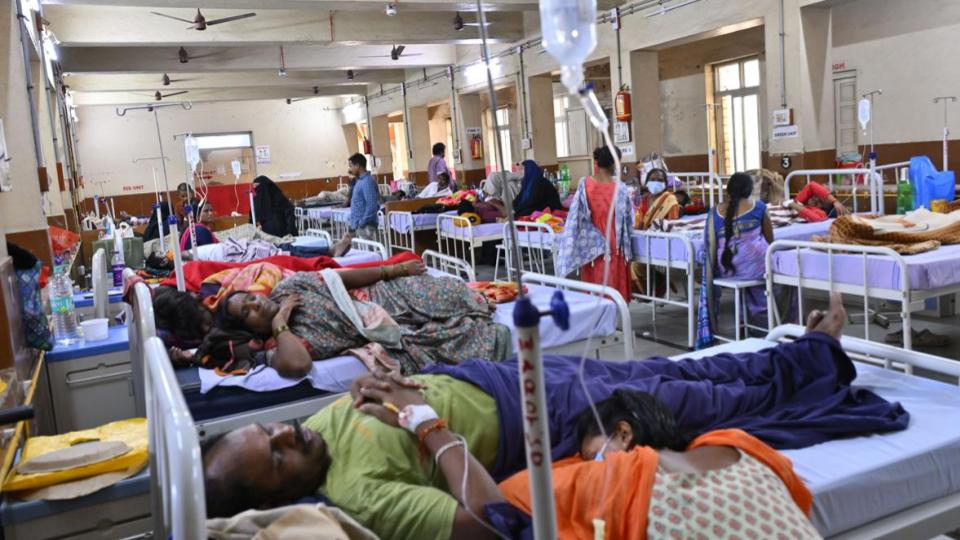
The WHO stated that it is closely monitoring recent media reports from India regarding clusters of pediatric illnesses and deaths in Madhya Pradesh and Rajasthan.
These reports, which have emerged during the week of September 29, describe symptoms consistent with acute renal failure and acute encephalitis syndrome, with suspected links to the use of oral syrup medicines.
The WHO has not received any official information as to the source of the DEG contamination or if contaminated pharmaceutical
material has been identified, sources told news agency PTI.
material has been identified, sources told news agency PTI.
The global health agency has expressed deep concern over the developments and emphasised the potential risk of contaminated products being exported to other countries, particularly via unregulated channels and the regulatory gap in DEG/EG screening for domestically marketed medicines in India.
Earlier, the Drugs Controller General of India (DCGI) had urged drug controllers of all states and Union territories to ensure testing of raw materials and finished formulations of pharmaceutical products before releasing in them in the market in the wake of children deaths allegedly due to consumption of contaminated cough syrup in Madhya Pradesh.
In an advisory, the DCGI said that during recent inspections at manufacturing facilities and in the investigations of the drugs declared as not of standard quality, it was found that several manufacturers are not testing each batch of excipients and active ingredients for compliance with prescribed standards before use.
No Comments For This Post, Be first to write a Comment.
Most viewed from National
Most viewed from World
AIMIM News
Latest Urdu News
Most Viewed
May 26, 2020
Where should be the burial of the pilgrims martyred in the Saudi Arabia bus accident?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2025 Etemaad Daily News, All Rights Reserved.




































.jpg)
.jpg)
.jpg)


