COVID-19 Vaccine: Britain Launches Trial to Combine Pfizer, AstraZeneca Vaccines in Two-Shot Schedule
Thu 04 Feb 2021, 17:39:31
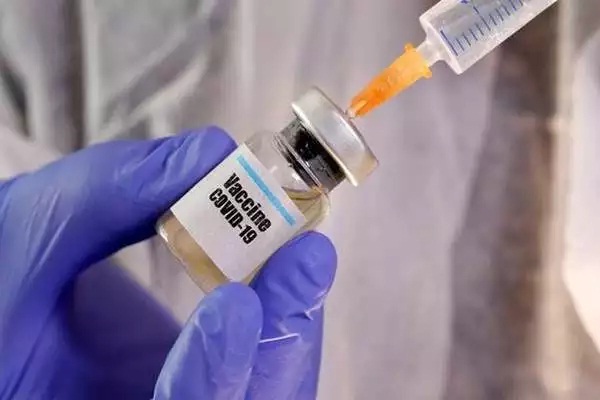
Britain launched a trial to assess the immune responses generated if doses of the COVID-19 vaccines from Pfizer Inc. and AstraZeneca Pharmaceuticals are combined in a two-shot schedule. The British researchers behind the trial said data on vaccinating people with the two different types of Coronavirus vaccines could help understanding of whether shots can be rolled out with greater flexibility around the world. Initial data on immune responses are expected to be generated around June.
The trial will examine the immune responses of an initial dose of Pfizer vaccine followed by a booster of AstraZeneca’s, as well as vice versa, with intervals of
four and 12 weeks.
four and 12 weeks.
Recruitment for the study starts on Thursday, with over 800 participants expected to take part, the researchers said. That makes it much smaller than the clinical trials that have been used to determine the efficacy of the vaccines individually.
The trial is looking to recruit people over the age of 50 who may be at higher risk than younger people and have not been vaccinated already.
AstraZeneca’s shot is also being tested in combination with Russia’s Sputnik V vaccine and the British drug maker’s research chief has said more studies on combining vaccines should be done.
No Comments For This Post, Be first to write a Comment.
Most viewed from National
Most viewed from World
AIMIM News
Latest Urdu News
Most Viewed
May 26, 2020
Is it right to exclude Bangladesh from the T20 World Cup?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2026 Etemaad Daily News, All Rights Reserved.























.jpg)












.jpg)
.jpg)
.jpg)


