Bharat Biotech announces Covaxin safe for children aged 2-18 years
Fri 17 Jun 2022, 21:37:23
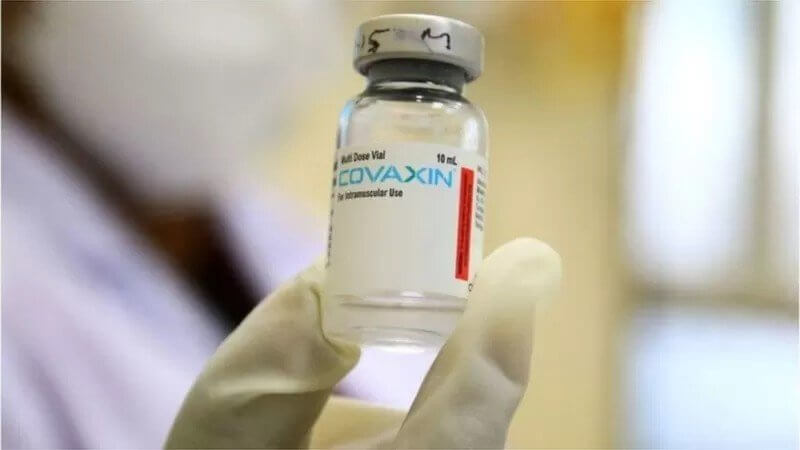
Bharat Biotech International Limited (BBIL) on Thursday announced that Covaxin, its whole-virion inactivated COVID-19 vaccine has proven to be safe, well-tolerated, and highly immunogenic in paediatric subjects in phase II/III study, said a press release.
The study has been accepted and published in Lancet Infectious Diseases, a peer-reviewed high-impact factor journal, added the statement.
Bharat Biotech conducted phase II/III, open-label, and multicentre studies to evaluate the safety, reactogenicity, and immunogenicity of COVAXIN in healthy children and adolescents in the 2-18 years of age group. The clinical trial conducted in the pediatric population between June 2021 to September 2021 has shown safety, less reactogenic, and robust immunogenicity. The data
was submitted to the Central Drugs Standard Control Organisation (CDSCO) in October 2021 and received a nod for emergency use in children aged 6-18years.
was submitted to the Central Drugs Standard Control Organisation (CDSCO) in October 2021 and received a nod for emergency use in children aged 6-18years.
Dr Krishna Ella, Chairman and Managing Director, Bharat Biotech, said, "Safety of the vaccine is critical for children and we are glad to share that COVAXIN has now proven data for safety and immunogenicity in children. We have now achieved our goal of developing a safe and efficacious COVID-19 vaccine for adults and children, for primary immunization and booster doses, and making COVAXIN a universal vaccine. It has proven to be a highly safe vaccine based on data from more than 50 million doses administered to children in India. Vaccines are a great preventive tool; the power of vaccines can only be harnessed if used prophylactically."
No Comments For This Post, Be first to write a Comment.
Most viewed from National
Most viewed from World
AIMIM News
Latest Urdu News
Most Viewed
May 26, 2020
Is it right to exclude Bangladesh from the T20 World Cup?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2026 Etemaad Daily News, All Rights Reserved.



































.jpg)
.jpg)
.jpg)


