India’s first intranasal Covid vaccine trials completed by Bharat Biotech
Tue 16 Aug 2022, 12:55:23
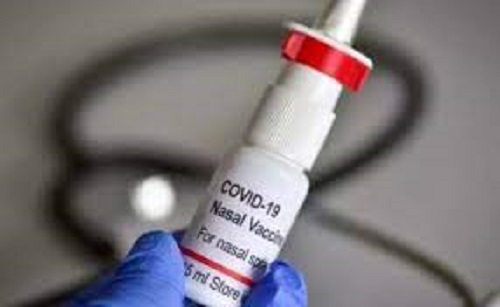
Bharat Biotech completed clinical development for Phase III trials and booster doses for India’s first intranasal Covid vaccine – BBV154. Two separate and simultaneous clinical trials were conducted to evaluate BBV154 as a primary dose (2-dose) schedule; and a heterologous booster dose for subjects who have previously received two doses of the two commonly administered covid vaccines in India.
Data from both Phase III human clinical trials have been submitted for approval to National Regulatory Authorities, it said. BBV154 (intra nasal vaccine) has proven to be safe, well-tolerated, and immunogenic in subjects in controlled clinical trials, it said.
This vaccine candidate was evaluated earlier in phase I and II clinical trials with successful results. BBV154 has been specifically formulated to allow intranasal delivery. In addition, the nasal delivery system has been designed and developed to be cost-effective in low and middle-income countries.
BBV154 was developed in partnership with Washington University St Louis, which had designed and developed the recombinant adenoviral vectored constructs and evaluated them in preclinical studies for efficacy.
Product development related to preclinical safety evaluation, large-scale manufacturing scale-up, formulation, and delivery device development including human
clinical trials were conducted by Bharat Biotech. The Government of India partly funded product development and clinical trials through the Department of Biotechnology’s Covid Suraksha programme.
clinical trials were conducted by Bharat Biotech. The Government of India partly funded product development and clinical trials through the Department of Biotechnology’s Covid Suraksha programme.
Two separate and simultaneous clinical trials were conducted to evaluate BBV154 as a primary dose (2-dose) schedule and a heterologous booster dose for subjects who have previously received 2 doses of the two commonly administered Covid vaccines in India.
“On this 75th Independence Day, we are proud to announce successful completion of clinical trials for BBV154 intranasal vaccine. This is yet another achievement for the multidisciplinary teams at Bharat Biotech. If approved, this intranasal vaccine will make it easier to deploy in mass immunisation campaigns with an easy to administer formulation and delivery device. Vectored vaccines also enable faster development of targeted vaccines in response to emerging variants of concern. We hereby thank the volunteers, investigators, and clinical trial personnel for all their efforts,” said Suchitra K Ella, Joint Managing Director, Bharat Biotech.
BBV154 is stable at 2-8°C for easy storage and distribution. Bharat Biotech has established large manufacturing capabilities at multiple sites across India, including Gujarat, Karnataka, Maharashtra and Telangana, with operations pan India.
No Comments For This Post, Be first to write a Comment.
Most viewed from Hyderabad
Most viewed from World
AIMIM News
Latest Urdu News
Most Viewed
May 26, 2020
Is it right to exclude Bangladesh from the T20 World Cup?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2026 Etemaad Daily News, All Rights Reserved.










.jpg)

























.jpg)
.jpg)
.jpg)


