Novo Nordisk set to launch Ozempic in India
Mon 29 Sep 2025, 20:59:33
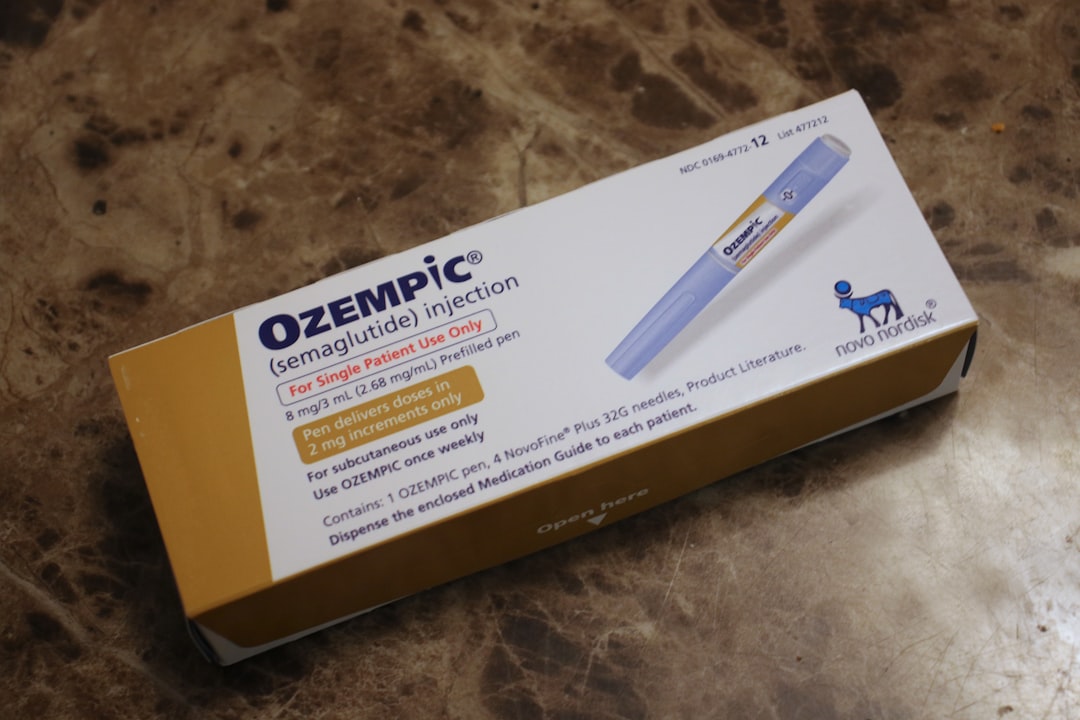
Danish drugmaker Novo Nordisk is set to launch Ozempic (semaglutide) in India, a move that will benefit patients suffering from Type 2 Diabetes in the country, says India Head, Vikrant Shrotiya, in an exclusive conversation with India Today.
Ozempic is a once-a-week injectable brand that was originally approved to manage type 2 diabetes, but was seen as one of the most sought after drugs given its benefits in weight management.
Speaking to India Today, Shrotiya said, “With the highly anticipated launch expected soon, Novo Nordisk will now complete its semaglutide portfolio in India, providing a range of treatments to address diverse patient needs— oral semaglutide for diabetes, advanced obesity management through Injectable”
Ozempic was first approved by the US FDA in 2017. The company has by far not given out the details of the costing of the medicine. Earlier this year, it got approval to sell WeGovy, the drug for obesity management.
Novo Nordisk is set to close its patent expiry for semaglutide in March next year, which is expected to unleash a wave of generic versions from leading Indian drugmakers. “With our patents expiring in 2026, so many more players would enter the market. The competitive intensity itself could bring down the cost of medicines, at least, amongst the generics,” added Shrotiya.
“We are not a company that just believes in the pricing part of it. We also strongly believe in engagement with our patients. The availability of medicine, trust and reliability are an important part of our business. The quality of medicines is of paramount importance," he
said.
said.
On the decision of the World Health Organization to add Ozempic to its list of essential medicines, Nova Nordisk said, this indicates the “relevance of the medicine."
“When WHO puts it out as a part of an essential medicine, it just goes to show how much value it brings to people with diabetes and obesity,” Shrotiya made it clear, adding that sometimes you hear about the kind of hue and cry semaglutide creates. It just takes away from that, the relevance of the drug gets established. "It showcases the confidence amongst regulators, doctors and health care providers."
Weight loss medicines have got bad press because reports that indicate side effects that are increasingly difficult to manage.
India has a large population of patients with type 2 diabetes. 101 million people suffer from this disease and another 254 million (and counting) are suffering from obesity. The numbers have doubled in the last decade, making weight management a serious matter.
“Obesity will continue to be an issue for the world. The journey of GLP-1 has started in India. It has to reach far and wide, in every corner of the country. Obesity is not just an urban India problem any more. The introduction of Ozempic is a welcome change, and I am sure this will help bring more formulary inclusions in government bodies, treating bodies and insurance,” Shrotiya said.
Injectable semaglutide indicated for Type 2 diabetes also lowers the risks of major adverse cardiovascular events (MACE) and chronic kidney disease, positioning it as a well-rounded therapy for patients.
No Comments For This Post, Be first to write a Comment.
Most viewed from Health
AIMIM News
Latest Urdu News
Most Viewed
May 26, 2020
Should there be an India-Pakistan cricket match or not?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2026 Etemaad Daily News, All Rights Reserved.






















.jpg)
.jpg)
.jpg)


