COVID-19 vaccine: Serum Institute signs deal with Gates Foundation; to price vaccine below ₹250 per dose
Fri 07 Aug 2020, 16:13:15
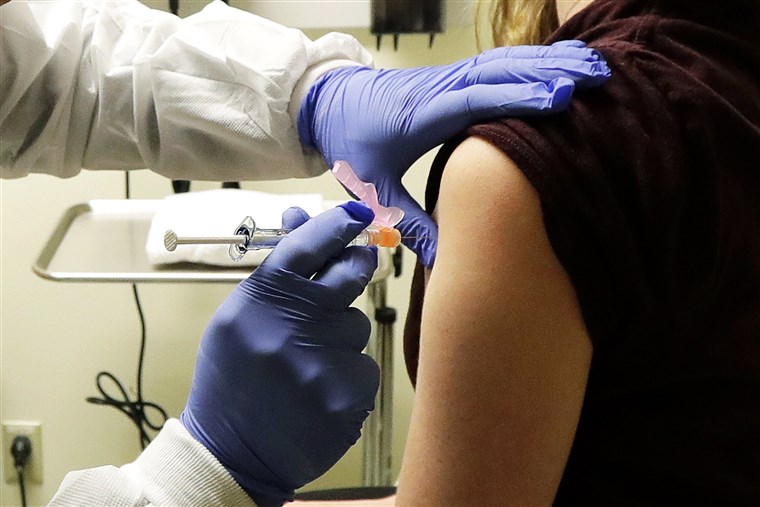
Serum Institute of India (SII) on Friday said the Bill & Melinda Gates Foundation will provide at-risk funding of $150 million to manufacture 100 million doses of COVID-19 vaccines for India and low-and-middle income countries. Indian drugmaker has earlier joined hands with Astra Zeneca and Novavax to develop their COVID-19 candidates. Under this agreement, Pune-based firm can charge a maximum of $3 per dose for the two COVID-19 vaccines. The vaccine maker will get the funding from the Gates Foundation through international vaccine alliance GAVI.
"The funding will support at-risk manufacturing by SII for candidate vaccines from AstraZeneca and Novavax," the drugmaker said. The vaccines will be available for procurement if they are successful in attaining full licensure and World Health Organisation prequalification, the company further added.
Novavax Inc said on Wednesday it has entered a supply and license agreement with the Serum Institute of India for the development and commercialisation of its COVID-19 vaccine candidate.
SII, the world’s largest vaccine manufacturer by number of doses produced and sold globally, will have exclusive rights for the vaccine in India during the term of the deal and non-exclusive rights during the "Pandemic Period" in all countries other than those designated by the World Bank as upper-middle or high-income countries.
On Tuesday, Novavax reported that its
experimental COVID-19 vaccine produced high levels of antibodies against the novel coronavirus in a small, early-stage clinical trial. The company said it could start a large pivotal Phase III trial as soon as late September.
experimental COVID-19 vaccine produced high levels of antibodies against the novel coronavirus in a small, early-stage clinical trial. The company said it could start a large pivotal Phase III trial as soon as late September.
"Given our involvement in Novavax on the improvement of a jungle fever immunization, we know the intensity of their antibody advancements," said Adar Poonawalla, CEO of Serum Institute of India.
Prior, British-Swedish firm Astra Zeneca has collaborated with Serum Institute of India to fabricate COVID-19 antibody competitor created by the University of Oxford. The Drugs Controller General of India (DCGI) has endorsed the immunization creator to lead Phase II and III clinical preliminaries of Oxford University-Astra Zeneca COVID-19 antibody in India.
Oxford's COVID-19 vaccine has shown a positive result in its initial trial. According to a report published in the British medical journal, The Lancet, the COVID-19 vaccine produced a dual immune response in people aged 18 to 55.
On production of the Oxford's COVID-19 vaccine, Poonawalla added, "Keeping in mind the pandemic situation, we have two dedicated facilities to produce millions of doses of the COVID-19 vaccine, while withholding vast production of other products."
The company has already manufactured around 2-3 million doses of the Oxford COVID-19 vaccine candidate for getting the process correct.
No Comments For This Post, Be first to write a Comment.
Most viewed from Coronavirus Updates
Most viewed from Health
AIMIM News
Latest Urdu News
Most Viewed
May 26, 2020
Is it right to exclude Bangladesh from the T20 World Cup?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2026 Etemaad Daily News, All Rights Reserved.

































.jpg)
.jpg)
.jpg)


