World's first intranasal vaccine by Bharat Biotech gets approval
Mon 28 Nov 2022, 22:16:49

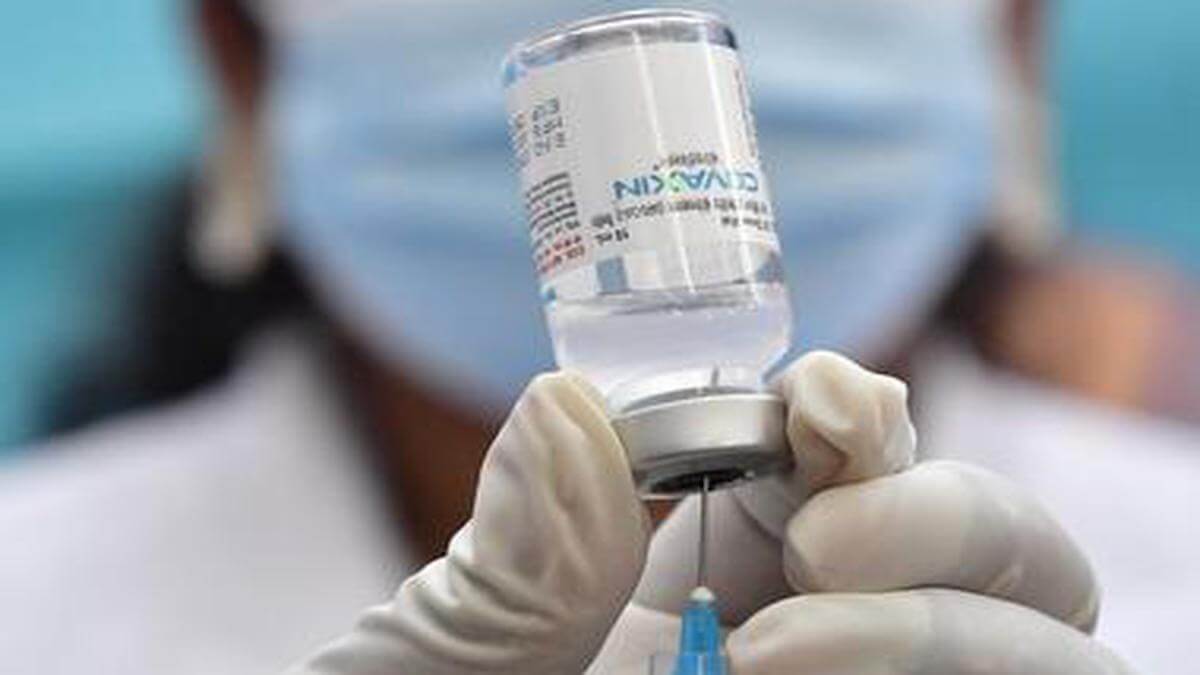
iNCOVACC, the world's first intranasal vaccine by Bharat Biotech has received both primary series and Heterologous booster approval, the Hyderedab-based company said on Monday.
In a release regarding the same, Bharat Biotech said, iNCOVACC (BBV 154), has received approval from the Central Drugs Standard Control Organisation (CDSCO) under Restricted Use in Emergency Situation for ages 18 and above, in India, for heterologous booster doses.
The intranasal vaccine had earlier received approval under Restricted Use in Emergency Situation for ages 18 and above for the primary 2-dose schedule. Phase III trials were conducted for safety, immunogenicity in~3100 subjects, at 14 trial sites across India.
Meanwhile, the heterologous booster dose studies were conducted for safety and
immunogenicity in ~875 subjects, with BBV154 intranasal vaccine administered post 2 doses of the two commonly administered COVID-19 vaccines. The trials were conducted at 9 trial sites across India.
immunogenicity in ~875 subjects, with BBV154 intranasal vaccine administered post 2 doses of the two commonly administered COVID-19 vaccines. The trials were conducted at 9 trial sites across India.
Speaking on the recent development, Dr. Krishna Ella, Chairman & Managing Director, Bharat Biotech, said, “This is a great achievement for us and the global scientific community to enable nasal administration of COVID vaccines. Despite the lack of demand for COVID vaccines, we continued product development in intranasal vaccines to ensure that we are well-prepared with platform technologies for future infectious diseases.
“We thank the Ministry of Health, CDSCO, Dept of Biotechnology, Govt of India, Technology Development Board, and Washington University, St. Louis, for their support and guidance, " he added.
No Comments For This Post, Be first to write a Comment.
Most viewed from
Most viewed from Health
AIMIM News
Latest Urdu News
Most Viewed
May 26, 2020
Which cricket team will win the IPL 2024?
Latest Videos View All
Like Us
Home
About Us
Advertise With Us
All Polls
Epaper Archives
Privacy Policy
Contact Us
Download Etemaad App
© 2024 Etemaad Daily News, All Rights Reserved.














.jpg)